calcium carbonate chips and hydrochloric acid|Rate of Reaction : Cebu An investigation of the reaction between marble chips and hydrochloric acid: Marble chips, calcium carbonate (CaCO 3) react with hydrochloric acid (HCl) to produce carbon dioxide gas. Calcium chloride solution is . Welcome to the HP Care Pack Central 2.0 site which allows you to quickly and easily identify Care Pack Services for your HP computing and printer products. Select Geography: Japan Clear. Care Packs listed are available in all countries/regions selected. Enter Hw/Sw number, serial number, product model name or Care Pack SKU .
PH0 · The effect on mass loss of a reaction between hydrochloric acid
PH1 · Rate of Reaction
PH2 · Practical: Effect of Surface Area on Rate of Reaction
PH3 · Effect of surface area on rates of reaction
PH4 · Core Practical: Investigating Rate of Reaction
PH5 · Calcium carbonate, hydrochloric acid, and their
PH6 · Calcium carbonate puriss., meets analytical specification of Ph.
PH7 · Calcium Carbonate and Hydrochloric Acid Reaction
PH8 · CaCO3 + HCl
PH9 · 3:15 practical: investigate the effect of changing the surface area of
PH10 · 3:15 practical: investigate the effect of changing the
Adult Content. This site contains adult content. If you are under 18 years of age or have not reached the legal adult age in your area or it is illegal to view adult content in your area, please leave the site immediately.
calcium carbonate chips and hydrochloric acid*******: This study demonstrates the efficacy of Ca(OH)(2)-treated cypress biochar, rich in calcium carbonate, in immobilizing heavy metals and enhancing radish growth in contaminated soils, offering sustainable solutions for soil remediation (Shah et al., 2024).
As the marble chips of calcium carbonate was added to the hydrochloric acid, the chips instantly starting fizzing which lead to the production of large bubbles.The Reaction of Calcium Carbonate and Hydrochloric Acid. At GCSE level, this reaction is a standard experiment to see the effect of changing the surface area of a reactant on .
If you had a large marble chip, all the calcium carbonate in the middle of the chip can't react with the acid, because the acid can't get at it. But if you had the same mass of smaller chips, you have opened up a much larger .calcium carbonate chips and hydrochloric acidAn investigation of the reaction between marble chips and hydrochloric acid: Marble chips, calcium carbonate (CaCO 3) react with hydrochloric acid (HCl) to produce carbon dioxide gas. Calcium chloride solution is .Investigating the effect of different size marble chips on the rate of reaction between calcium carbonate and hydrochloric acid Method: Add a fixed volume of hydrochloric .Calcium carbonate, hydrochloric acid, and their interaction. How CaCO₃ reacts with HCl. [Deposit Photos] Let’s talk a little about the interaction between hydrochloric acid and calcium carbonate, and the nature of .When a gas is produced in a reaction it usually escapes from the reaction vessel, so the mass decreases. This can be used to measure the rate of reaction. For example, the .Rate of Reaction This chemistry video tutorial explains how to predict the products of the reaction between Calcium Carbonate and Hydrochloric Acid. It also explains how to w.
Investigating the effect of different size marble chips on the rate of reaction between calcium carbonate and hydrochloric acid. . Add a fixed volume of hydrochloric acid into a conical flask; Use a delivery tube to connect this flask to an inverted measuring cylinder; Add marble chips into the conical flask and close the bung;
calcium carbonate chips and hydrochloric acid Rate of Reaction A student investigated the rate of reaction between calcium carbonate (marble chips) and hydrochloric acid. The student used the apparatus shown in Figure 1 . The student: •€€€€€€€€recorded the volume of gas collected every 5 seconds •€€€€€€€€repeated the experiment using hydrochloric acid at different .Q6. A student investigated the rate of reaction between calcium carbonate (marble chips) and hydrochloric acid. The student used the apparatus shown in Figure 1. The student: Recorded the volume of gas collected every 5 seconds Repeated the experiment using hydrochloric acid at different temperatures. The equation for the reaction is:
Space-filling model of part of the crystal structure of calcium carbonate, CaCO₃ [Wikimedia] CaCO₃ is a widespread compound found in chalk, lime, marble, and more. This substance is a crucial pillar of human life – it is used in construction, to manufacture paper and plastic, and in many other spheres.
How does reaction rate change with increasing surface area of calcium carbonate in hydrochloric acid ? Hypothesis. With increasing surface area of marble chips the reaction rate will also increase. Justification. For the same mass of the calcium carbonate, powder has a larger surface area than marble chips. As the3 A student investigates the rate of the reaction between marble chips (calcium carbonate) and dilute hydrochloric acid. She is given a bottle containing hydrochloric acid labelled 100%. She uses this method to find out how changing the concentration of the acid affects the rate of reaction. x add some marble chips to a conical flaskStudent A investigated the rate of reaction between calcium carbonate (marble chips) and hydrochloric acid. The student collected 7.5 cm3 of gas produced after 15 seconds. Student B investigated the rate of reaction between magnesium and hydrochloric acid. The student collected 17 cm3 of gas produced after 10 seconds. One such example of a reaction is the reaction which occurs when calcium carbonate is added to hydrochloric acid. Calcium carbonate reacts with hydrochloric acid to produce carbon dioxide gas, water and calcium chloride solution, as shown by the equation: . Add a known mass of large marble chips to the acid and immediately .

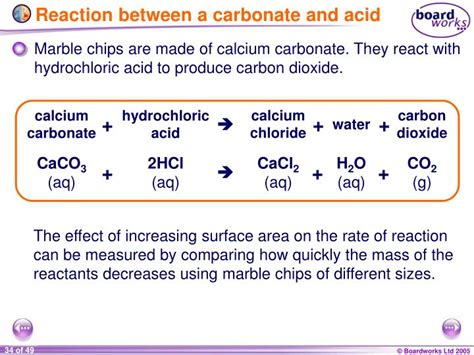
small marble chips, and dilute hydrochloric acid, HC l. The equation for the reaction is given below. CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g) FA 1 is approximately 1.0 g calcium carbonate, CaCO3. FA 2 is approximately 2 mol dm–3 hydrochloric acid, HCl. (a) Method Read through the whole method before starting any practical work.0 9 Marble chips are mainly calcium carbonate (CaCO 3). A student investigated the rate of reaction between marble chips and hydrochloric acid (HCl). Figure 9 shows the apparatus the student used. Figure 9 . 0 9 . 1 Complete and balance the equation for the reaction between marble chips and hydrochloric acid. [2 marks] + → CaCl 2 + . These are instructions for preparing carbon dioxide gas from calcium carbonate and hydrochloric acid. It is not a difficult process. Menu. Home. Science, Tech, Math Science Math Social .
Reaction: Hydrochloric acid + Calcium carbonate → Calcium chloride + water + carbon dioxide. 2HCl + CaCO3 → CaCl2+ H2O + ↑ CO2 . The concentration of Acid. Use 2g of marble chips for this experiment. Add .Add calcium carbonate powder to a conical flask. Pour hydrochloric acid into the flask, mix, and immediately attach a gas syringe. Measure how much gas has been produced every 20 seconds, and record in a table. Repeat these steps with other concentrations of hydrochloric acid as well (keep the volume the same).
Add calcium carbonate powder to a conical flask. Pour hydrochloric acid into the flask, mix, and immediately attach a gas syringe. Measure how much gas has been produced every 20 seconds, and record in a table. Repeat these steps with other concentrations of hydrochloric acid as well (keep the volume the same).
Title: Effect of Acid Concentrations on Reaction Rates, Student Experiment Report Aim: To determine the effect of concentration on the rate of reaction using the calcium carbonate (CaCO3) chips (measured to 1 gram) and hydrochloric acid (2.0M) by measuring the time to dissolve 1 gram of CaCO3 chips with varying dilutions of hydrochloric acid and water.
Add some marble chips to hydrochloric acid in a conical flask with a side arm and draw attention to the gas evolved. Issue mini whiteboards. . The amount of calcium carbonate is twice what it is in experiment 1 so that the volume of carbon dioxide will also be twice that produced in experiment 1. When we add Hydrochloric acid to Calcium carbonate, the following chemical reaction takes place:HCl (aq) + CaCO3 (s) CaCl2 (aq) + CO2 (g) + H2O (l)We can s.
The original experiment used calcium carbonate chips, calcium carbonate powder and hydrochloric acid to determine how surface area effects the rate of the reaction. The rate of the reaction was measured using the scales and the time was recorded using a stopwatch. 4.3 Modifications
"Family Strokes" Family Game Night (TV Episode 2015) cast and crew credits, including actors, actresses, directors, writers and more. . What to Watch Latest Trailers IMDb Originals IMDb Picks IMDb Spotlight IMDb Podcasts. . See agents for this cast & crew on IMDbPro Cast Dane Cross Sindy Lange Ryan McLane Alina West .
calcium carbonate chips and hydrochloric acid|Rate of Reaction